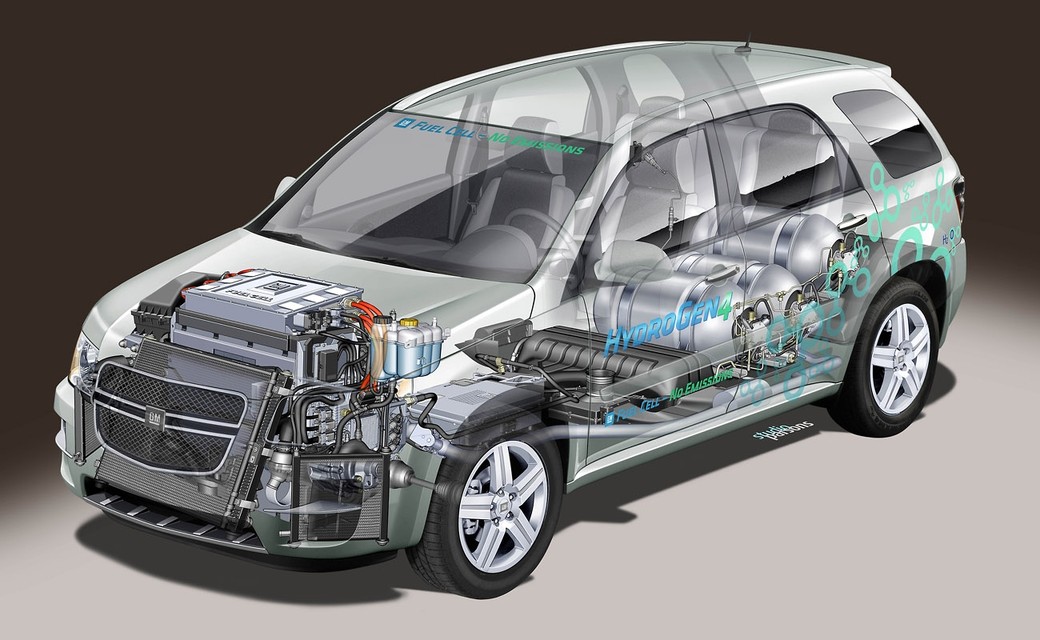
Putting the “Science” in “Science Fiction”: Hydrogen Power, Part 2
In last week’s blog, I talked about the many possibilities offered by using combustible hydrogen as a power source for cars and other vehicles. Hydrogen holds a great deal of promise for use in this manner, but there is another entirely different way of drawing energy from hydrogen. This method uses a device called a fuel cell in order to produce electricity from hydrogen.
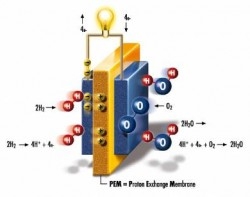
There are several different types of fuel cells and each has possible applications, but I will focus on polymer exchange membrane fuel cells, as these are the most likely to see everyday use in cars and other vehicles. A fuel cell of this type consists of two electrodes connected by a catalyst and a proton exchange membrane, which acts as an electrolyte. The fuel cell pumps in hydrogen atoms from one side and oxygen atoms from the other. The hydrogen atoms are forced through the catalyst, which usually consists of platinum, and is separated into electrons and positively-charged hydrogen ions. The electrolyte is made up of a material that only conducts positively-charged ions, forcing the hydrogen atoms through, while leaving the electrons behind.

The electrons are channeled through an external wire to the other side of the electrolyte, creating electrical energy. When the electrons and hydrogen ions have reached the other side of the electrolyte, they combine with the oxygen atoms to create water molecules, which then leave the fuel cell and are either collected or allowed to escape into the air. A fuel cell of this type is clean, has no moving parts, and is extremely efficient. Counting the energy that is lost from creating the hydrogen fuel from water in the first place, a current hydrogen concept car has roughly 60% energy-efficiency. In comparison, a conventional gasoline car has 20% energy-efficiency, not counting the energy that is lost in extracting and processing fossil fuels. An entirely electric car has either the same or significantly less energy-efficiency than a hydrogen car, depending on what type of generator produced the electricity in the first place, leaving hydrogen fuel cells as a serious contender for the future of vehicle engines.

Although fuel cells and hydrogen fuel have many advantages over conventional engines, hurdles must still be overcome before this technology can become widely adopted. Besides the general issues about hydrogen power I mentioned in my last blog, there are a few issues unique to fuel cells. A major one is cost, as current fuel cells require rare metals like platinum to operate. Current growth in nanotechnology offers a potential solution to this problem, but until one is found, fuel cells will be far more expensive than conventional engines. Durability and hydration are linked. In order to operate, a fuel cell’s electrolyte must be kept damp, which can be difficult to achieve when it is operating for long periods of time. This fact, combined with the high temperatures such a fuel cell is likely to reach, could lead to degradation of the cell as it is used, which is another issue that will have to be addressed before wide-scale manufacturing can begin.
Although fuel cells are not the total solution to the world’s need for green energy, they certainly offer the potential for a green future. As the necessary technology improves and energy generation moves from fossil fuels to renewable sources like wind and solar, hydrogen fuel cells and other new energy technologies will help to solve the environmental, political and economic problems caused by our reliance on (nay, our addiction to) fossil fuels.
Top Photo: renewpowerzone.blogspot.ca








